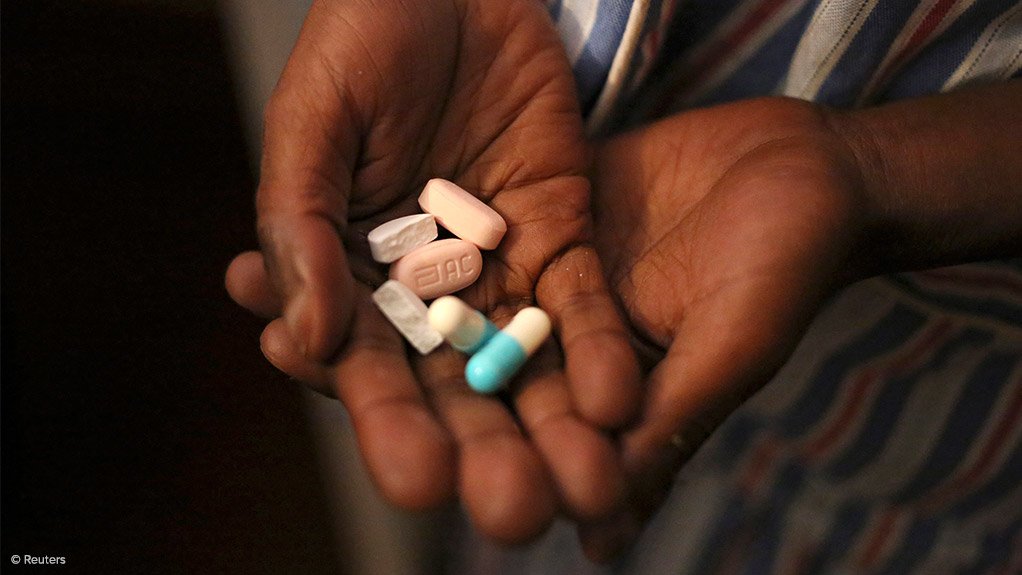
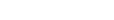Parliamentary Portfolio Committee on Health chairperson Faith Muthambi is urging greater transparency and accountability in the process of awarding of antiretroviral drugs (ARVs) tenders by the Department of Health (DoH).
The committee expressed concerns over an apparent lack of due diligence by the DoH when awarding tenders to Barrs Pharmaceuticals Industries and Innovata Pharmaceuticals, which were placed under business rescue in December 2025.
The committee questioned the impact of the two suppliers being unable to meet their contractual obligations and the extent of the problem, as well as the contingency plans the department had in place, and if due diligence was conducted on the suppliers prior to their receiving the tender.
Muthambi noted with concern that due diligence appeared to have been desktop-based, limiting the accuracy of local assessments.
The DoH indicated that it was procuring at least 70% of its ARVs from local manufacturers, however, committee members questioned this, estimating that 70% of ARVs were imported, while only 30% were produced domestically.
“It would appear that the country has at least four-million monthly patient capacity for Tenofovir Dolutegravir and Lamivudine (TDL) and yet less than 50% of this has been awarded domestically,” she said.
Muthambi explained that this assessment was based on several factors, including referencing TDL, which accounts for 80% of the contract value.
“For the 84s pack size, members noted that Emcure (India) won 15% of the tender, but trade data from Indian customs indicates that Emcure imports its ARVs into South Africa.
“In the last three-year contract, which ended two months ago, Emcure imported $43-million (R1.6-billion) worth of ARVs, yet the department lists Emcure as local,” Muthambi pointed out.
She highlighted that the department lists Barrs, Innovata, Aurobindo and Viatris as local manufacturers, despite evidence that they are importing ARVs into South Africa.
Furthermore, Barrs, having gone into business rescue, had been acquired by Hetero, which would import ARV products into South Africa, she said.
The committee wants the department to account for the discrepancy in the requirement that a minimum 70% be locally procured, when import data for the mentioned suppliers indicates otherwise.
The committee said it sought documentation proving that these manufacturers were indeed producing ARVs in South Africa.
“Additionally, members inquired why local producers like Sunpharma, Cipla and Adcock Ingram were excluded in favour of importers, and why the department did not maximise the capacities of both Aspen and Cipla, which could provide three-million monthly patient treatments, when both companies are competitive,” Muthambi said.
The committee wants a breakdown of how the DoH conducts its due diligence, particularly in confirming the shareholding of these companies and verifying whether manufacturers claiming to produce ARVs in South Africa are actually doing so.
EMAIL THIS ARTICLE SAVE THIS ARTICLE ARTICLE ENQUIRY FEEDBACK
To subscribe email subscriptions@creamermedia.co.za or click here
To advertise email advertising@creamermedia.co.za or click here